Our Science
|
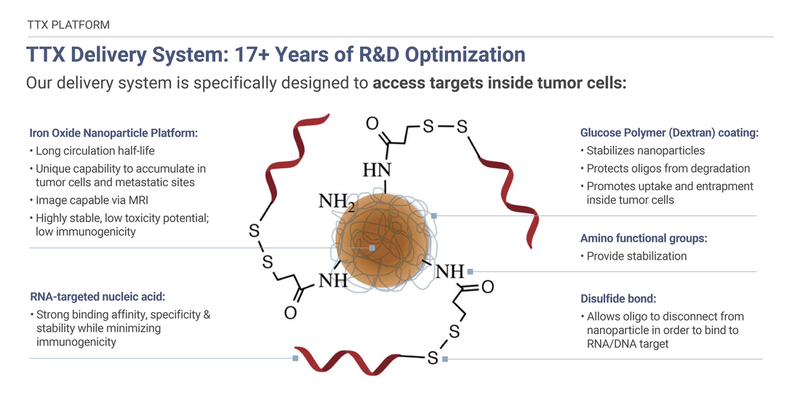
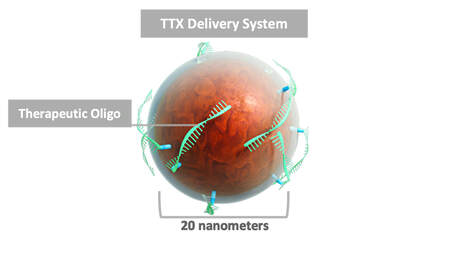
TransCode Therapeutics believes it has overcome one of the greatest challenges facing targeted therapy for cancer- the challenge of delivering a functional therapeutic to the tumor while sparing healthy tissue. Our solution involves a proprietary delivery platform, called TTX, which is specifically designed to transport therapeutic payloads to tumors and metastases.
Our therapeutic delivery strategy employs nanoparticles extensively used in imaging that have been repurposed and optimized to efficiently deliver payloads to oncology targets. Preclinical evidence TTX has overcome delivery challenges:
Yoo B, et al. J Biomed Nanotechnol. 2014;10(6):1114-1122.; Yigit MV, et al. Pharm Res. 2012;29(5):1180-1188.; Weissleder R, et al. Radiology. 1991;181(1):245-249.
|
View or download additional information on the TTX delivery platform and how TransCode is decoding the major challenge of RNA delivery.
|