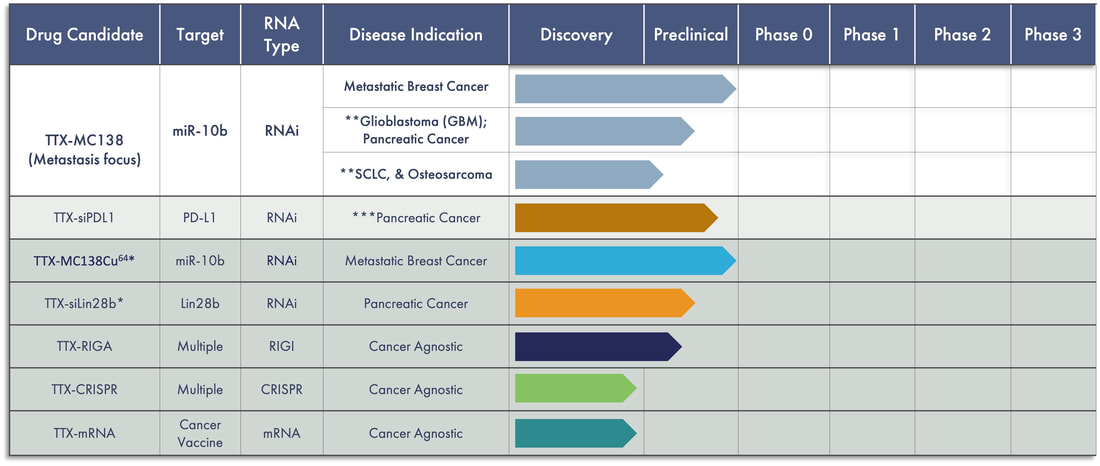
We have created a platform of drug candidates with the objective of improving patient outcomes. Our lead therapeutic candidate, TTX-MC138, is focused on treating metastatic cancer, which has been shown to be responsible for approximately 90% of all cancer deaths annually. We believe that TTX-MC138 has the potential to produce regression without recurrence in a range of cancers, including breast, pancreatic, ovarian and colon cancer, glioblastomas and others. Additionally, the Company’s other drug candidates, TTX-siPDL1, TTX-siLIN28b, TTX-RIGA, TTX-CRISPR and TTX-mRNA, focus on treating cancer using RNA technology made possible through its proprietary delivery platform.
Currently TransCode is continuing research on a variety of both diagnostic and therapeutic approaches targeting biomarkers broadly prevalent across numerous tumor types.
* TransCode signed Exclusive Option Agreements with The General Hospital Corporation, d/b/a Massachusetts General Hospital, or MGH, for TTX-siLin28b and 64Cu-TTX-MC138. Under these Options, TransCode has the right to negotiate a license for these candidates with MGH. TransCode’s decision will depend on the results of preclinical studies it plans to conduct as shown above.
PDAC: Pancreatic ductal adenocarcinoma
** Seeking Orphan designation status
***Received Orphan designation status from FDA
PDAC: Pancreatic ductal adenocarcinoma
** Seeking Orphan designation status
***Received Orphan designation status from FDA
Establishing Proof of Concept
As a next step, the company intends to advance TTX-MC138 to the clinic to be evaluated in human subjects suffering from late stage cancer. TransCode has received written guidance from the FDA in response to our pre-IND submission for our proposed first-in-human study and we are proceeding ahead under that guidance. Based on the results of this study, the exploratory IND will be withdrawn, and a full IND will be submitted to support a Phase I/II clinical trial intended to evaluate safety and dose/response and treatment of patients with metastatic cancer.
The FDA has not evaluated or approved TTX-MC138 and it is currently not available for patient use.